Concordare SuiteTM
Concordare is a web application that can both produce and read a digital protocol (Concordare’s proprietary CPTM). The suite can be used by site staff and protocol authors to enhance their clinical trial operations. Learn more about how the Concordare suite can add value to your objectives below.
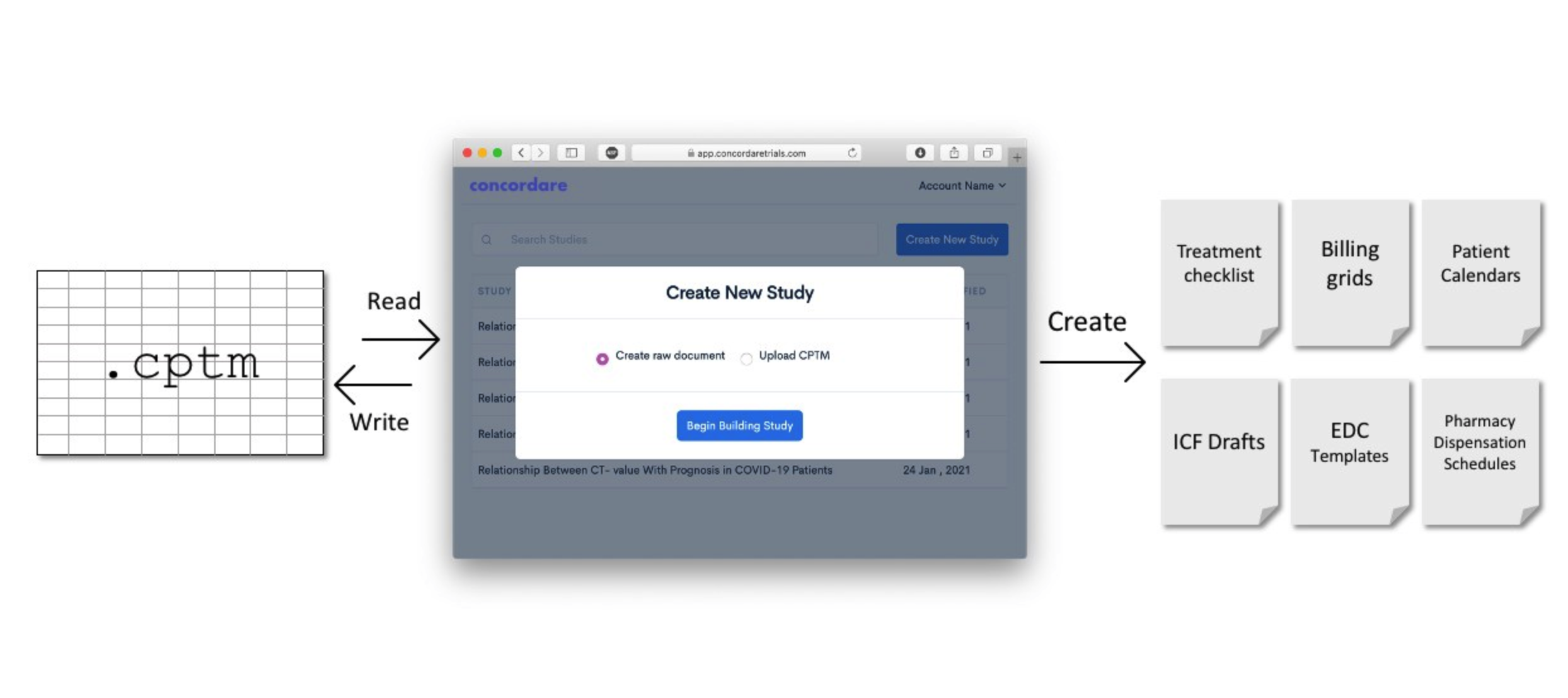
Concordare SuiteTM can be used right away, no plug-ins, no strategies, no pauses
Concordare SuiteTM can take a protocol at any phase of its trial lifecycle and digitize it within minutes. Once digitized, the protocol can be shared with unlimited stakeholders who can return to Concordare Suite to download powerful operational solutions.
Clinical site staff
Site staff of the key users of the Concordare SuiteTM. Using the suite, staff can quickly find details on a protocol and download many operational tools. Using Concordare leads to several benefits:
- Higher clinical trial volume capacity
- Enhanced patient tools for a better patient experience
- Easier study training and onboarding
- Lower chance of protocol and medical errors
- Enhanced protocol information lookup
- Decreased technical demand on clinical staff
Trial sponsors and protocol authors
Sponsors and protocol authors can immediately convert there protocols to a digital protocol using the Concordare SuiteTM. The digital protocol can immediately be distributed to operational stakeholders who can access them through the same suite and enhance their clinical trial operations. Concordare provides several value-adds for sponsors and investigators:
- Faster study start-up and patient enrollment
- Higher quality data due to lower protocol deviation rate
- Efficient site over-head and labor costs
- Smoother protocol amendment creation and implementation
- Easy protocol distribution and communication
- Stronger sponsor-site partnerships
The most important stakeholder: everyone
Faster drug discovery – trials meet goals faster, answering the question “does this treatment work?” even faster
